Los conjugados de colorantes iFluor® 440 son significativamente más brillantes que los correspondientes bioconjugados de DEAC u otros colorantes espectralmente similares (como SpectrumAqua).
Descripción
Los tintes iFluor® de AAT Bioquest están optimizados para marcar proteínas, en particular anticuerpos. Estos tintes son brillantes, fotoestables y tienen un enfriamiento mínimo de las proteínas. Pueden excitarse bien con las principales líneas láser de los instrumentos de fluorescencia (p. ej., 350, 405, 488, 555 y 633 nm).
Los tintes iFluor® 440 están diseñados para ser un reemplazo superior del tinte DEAC que tiene una solubilidad en agua extremadamente baja. iFluor® 440 SE es razonablemente estable y muestra buena reactividad y selectividad con los grupos amino de proteínas. En las mismas condiciones, los conjugados de colorantes iFluor® 440 son significativamente más brillantes que los correspondientes bioconjugados de DEAC u otros colorantes espectralmente similares (como SpectrumAqua), lo que hace que los conjugados iFluor® 440 sean mucho más sensibles.
| Catalogo | Producto | Presentación |
|---|---|---|
| AAT-1041 | iFluor® 440 succinimidyl ester | 1mg |
| AAT-71041 | iFluor® 440 succinimidyl ester | 100 ug |
| AAT-71519 | iFluor® 440 succinimidyl ester | 5mg |
| AAT-71569 | iFluor® 440 succinimidyl ester | 10mg |
Importante: Solo para uso en investigación (RUO). Almacenamiento: Congelación (< -15 °C). Minimizar la exposición a la luz.
Propiedades fisicas
| Peso Molecular | 692.83 |
| Disolvente | DMSO |
Espectro
Abrir en Advanced Spectrum Viewer
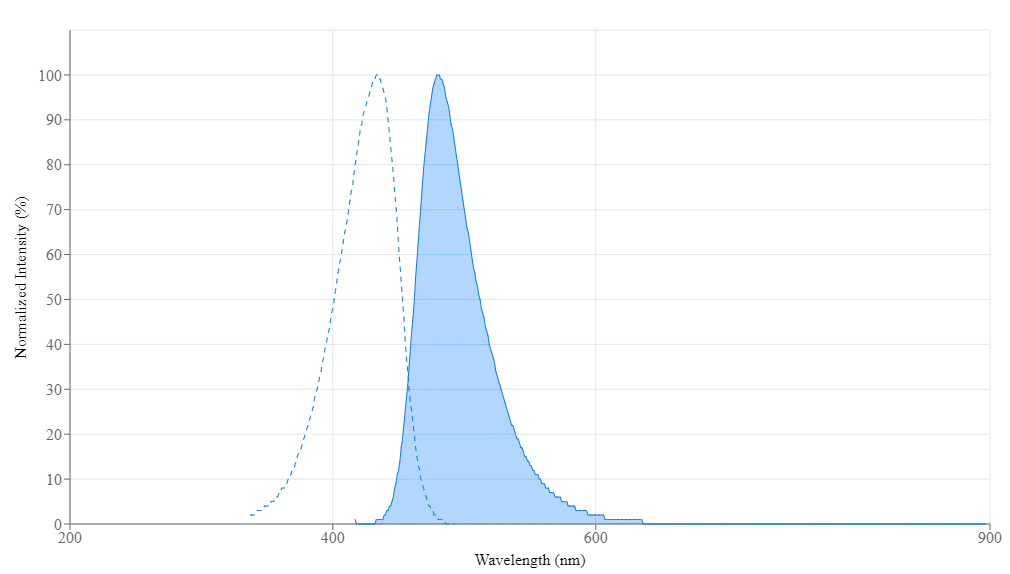
Propiedades espectrales
| Absorbancia (nm) | 430 |
| Factor de corrección (260 nm) | 0.352 |
| Factor de corrección (280 nm) | 0.229 |
| Coeficiente de extinción (cm -1 M -1) | 400001 |
| Excitación (nm) | 434 |
| Emisión (nm) | 480 |
| Rendimiento cuántico | 0.671 |
Calculadora
Preparación de la solución de stock común
Volumen de DMSO necesario para reconstituir la masa específica de succinimidil éster iFluor® 440 a la concentración dada. Tenga en cuenta que el volumen es solo para preparar la solución madre. Consulte el protocolo experimental de la muestra para conocer los buffers experimentales/fisiológicos apropiados.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 144.336 µL | 721.678 µL | 1.443 mL | 7.217 mL | 14.434 mL |
| 5 mM | 28.867 µL | 144.336 µL | 288.671 µL | 1.443 mL | 2.887 mL |
| 10 mM | 14.434 µL | 72.168 µL | 144.336 µL | 721.678 µL | 1.443 mL |
Imagen
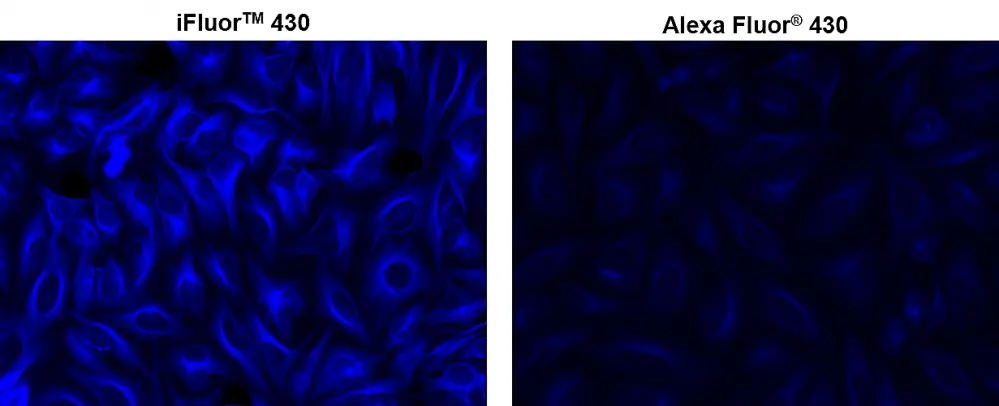
Figura 1. Las células HeLa se incubaron con antitubulina de ratón seguida de conjugado de IgG anti-ratón de cabra iFluorTM 430 de AAT (izquierda) o IgG anti-ratón de cabra conjugado con Alexa Fluor® 430 (derecha), respectivamente.
Productos Similares
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield | Correction Factor (260 nm) | Correction Factor (280 nm) |
| iFluor® 350 succinimidyl ester | 345 | 450 | 200001 | 0.951 | 0.83 | 0.23 |
| iFluor® 488 succinimidyl ester | 491 | 516 | 750001 | 0.91 | 0.21 | 0.11 |
| iFluor® 514 succinimidyl ester | 511 | 527 | 750001 | 0.831 | 0.265 | 0.116 |
| iFluor® 532 succinimidyl ester | 537 | 560 | 900001 | 0.681 | 0.26 | 0.16 |
| iFluor® 555 succinimidyl ester | 557 | 570 | 1000001 | 0.641 | 0.23 | 0.14 |
| iFluor® 594 succinimidyl ester | 588 | 604 | 1800001 | 0.531 | 0.05 | 0.04 |
| iFluor® 633 succinimidyl ester | 640 | 654 | 2500001 | 0.291 | 0.062 | 0.044 |
| iFluor® 647 succinimidyl ester | 656 | 670 | 2500001 | 0.251 | 0.03 | 0.03 |
| iFluor® 660 succinimidyl ester | 663 | 678 | 2500001 | 0.261 | 0.07 | 0.08 |
Referencias
Ver todas las 12 referencias: Citation Explorer
7-(Diethylamino)coumarin-3-carboxylic acid as derivatization reagent for 405 nm laser-induced fluorescence detection: A case study for the analysis of sulfonamides by capillary electrophoresis.
Authors: Wu, Chengxin and Sun, Yuanyuan and Wang, Yuanhang and Duan, Wenzhen and Hu, Jiangyue and Zhou, Lei and Pu, Qiaosheng
Journal: Talanta (2019): 16-22
Design, synthesis and perception of fluorescently labeled isoprenoid cytokinins.
Authors: Kubiasová, Karolina and Mik, Václav and Nisler, Jaroslav and Hönig, Martin and Husičková, Alexandra and Spíchal, Lukáš and Pěkná, Zuzana and Šamajová, Olga and Doležal, Karel and Plíhal, Ondřej and Benková, Eva and Strnad, Miroslav and Plíhalová, Lucie
Journal: Phytochemistry (2018): 1-11
Intracellular Uncaging of cGMP with Blue Light.
Authors: Agarwal, Hitesh K and Zhai, Shenyu and Surmeier, D James and Ellis-Davies, Graham C R
Journal: ACS chemical neuroscience (2017): 2139-2144
Wavelength-selective one- and two-photon uncaging of GABA.
Authors: Amatrudo, Joseph M and Olson, Jeremy P and Lur, G and Chiu, Chiayu Q and Higley, Michael J and Ellis-Davies, Graham C R
Journal: ACS chemical neuroscience (2014): 64-70
A water soluble fluorescent polymer as a dual colour sensor for temperature and a specific protein.
Authors: Inal, Sahika and Kölsch, Jonas D and Sellrie, Frank and Schenk, Jörg A and Wischerhoff, Erik and Laschewsky, André and Neher, Dieter
Journal: Journal of materials chemistry. B (2013): 6373-6381
Structures, synthesis, and human Nod1 stimulation of immunostimulatory bacterial peptidoglycan fragments in the environment.
Authors: Fujimoto, Yukari and Fukase, Koichi
Journal: Journal of natural products (2011): 518-25
Fluorescent agonists for the Torpedo nicotinic acetylcholine receptor.
Authors: Krieger, Florian and Mourot, Alexandre and Araoz, Romulo and Kotzyba-Hibert, Florence and Molgó, Jordi and Bamberg, Ernst and Goeldner, Maurice
Journal: Chembiochem : a European journal of chemical biology (2008): 1146-53
Antimicrobial activity of various cationic molecules on foodborne pathogens.
Authors: Conte, Mariachiara and Aliberti, Francesco and Fucci, Laura and Piscopo, Marina
Journal: World journal of microbiology & biotechnology (2007): 1679-83
A series of related nucleotide analogues that aids optimization of fluorescence signals in probing the mechanism of P-loop ATPases, such as actomyosin.
Authors: Webb, Martin R and Reid, Gordon P and Munasinghe, V Ranjit N and Corrie, John E T
Journal: Biochemistry (2004): 14463-71
Fluorescent coumarin-labeled nucleotides to measure ADP release from actomyosin.
Authors: Webb, M R and Corrie, J E
Journal: Biophysical journal (2001): 1562-9
Application Notes
iFluor® Dye Selection Guide
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
Abbreviation of Common Chemical Compounds Related to Peptides
Bright Tide Fluor™-Based Fluorescent Peptides and Their Applications In Drug Discovery and Disease Diagnosis
FITC (Fluorescein isothiocyanate)
FAQ
Are any of the cyanine dyes infrared?
Are coumarin dyes pH sensitive?
Are there any alternatives to BrdU (Bromodeoxyuridine)?
Are there any alternatives to Cy5?
Are there any alternatives to indocyanine green (ICG)?
AssayWise
A practical guide for use of PE and APC in flow cytometry
Calbryte™ series now available
Buccutite™ Fluorescent Protein and Tandem Dye Antibody Labeling Kits
Fundamentals of Flow Cytometry
ReadiUse™ Lyophilized Phycobiliproteins


